I happen to live in a rather arid region.
The lack of annual rainfall and shortage of readily available groundwater magnify the importance of water in a survival situation. While we all need water to survive, it is much easier to come up with it in an area where there is high rainfall or a lot of streams and rivers to get water from.
Even the subterranean groundwater here has a high salt and mineral content, making it hard to use for drinking and gardening.
This Device Easily Turns Air Into Water!
As a general rule of thumb, we shouldn’t drink sea water (saltwater) for survival. That’s because the salt content in seawater is about 35 parts per thousand. By comparison, human blood only has a salinity of 9 parts per thousand. What that means is that with seawater having such a high concentration of salt in it, drinking seawater will actually put salt into our bloodstream, not remove excess salt from it. When the salt level gets high enough, it will kill us.
Hence the old passage of poetry which reads, “Water, water everywhere and not a drop to drink” from Samuel Taylor Coleridge’s poem “the Rime of the Ancient Mariner.” Sailors have long known that drinking salt water would kill them, even if they didn’t understand the science behind it.
Yet today salt water is finding its way into municipal water supplies around the world. The tiny nation of Israel is the world leader in this science of seawater desalination, getting 55 percent of its domestic water from desalination. This has been critical to Israel’s modern growth, as the tiny nation is one of the driest on the face of the earth. Even here at home we are jumping on the bandwagon, with over 60 desalination plants in operation or under construction between the various coastal states.
There are two basic ways that these desalination plants work, by reverse osmosis or flash distillation. For our purposes, at this moment, how these plants work isn’t as important as the fact that they work. Both technologies are complex enough that they can’t be readily reproduced at home, at least not without buying the hardware to build the system and having the electrical power to operate them.
For reference, reverse osmosis water treatment is actually used in a number of homes, as a water purification method. You can buy reverse osmosis water purification units and install them under your kitchen sink, purifying your drinking water. This produces some of the cleanest water you can have in your home, almost as clean as distilled water; which historically is considered to be the cleanest water that can be produced.
The problem with reverse osmosis, from a survival point of view, is that it requires a large amount of electricity for the pumps that pressurize the water passed through the filter elements. So, unless you have the capacity of producing a large amount of electrical power, it is not usable for survival.
However, while neither of these methods of seawater reclamations or seawater desalination is practical in a survival situation; that doesn’t mean that we should ignore the idea of seawater desalination altogether. There are other, simpler means of distillation that can be used to make seawater drinkable, which could provide us with a virtually limitless supply of drinking water, if we happen to live close enough to the ocean to take advantage of it.
Basic Distillation
Basic distillation involves heating the water to the point of turning it into vapor, then condensing this water vapor back into a liquid state. Water turns to steam (water vapor) at 212°F (100°C), which is a fairly low temperature, compared to other materials. Other chemicals, minerals, salt and even the dead bodies of bacteria and viruses are left behind, because they don’t turn into vapor at that temperature.
Water vapor produced in this manner is then passed through a condenser, cooling the water and turning it back into a liquid. The water captured from the vapor is pure, because any impurities are left behind in the vessel that the water has been heated in.
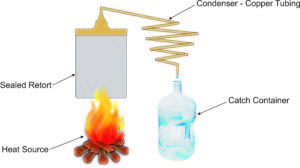
The still used for this process must be totally sealed, with the exception of the outlet for the product water. If it is not sealed, the water vapor will evaporate, rather than being condensed back into water.
Perhaps the easiest way to build a still is to start out with a pressure cooker or pressure canner. This provides a sealed retort, with a single outlet; the one used to allow steam to escape, regulating the pressure in the pressure cooker. We would need to remove the normal pressure regulator from that point, drill the hole out and thread it for the attachment of a brass fitting, so that we could attach the condenser tubing to it.
Copper tubing is the most common material used for making a condenser, as it is the second best heat conductor in existence. The only material which could perform better is silver, and buying enough silver tubing to make a condenser would be a bit too pricey to be practical.
As you can see in the diagram, the tubing used for the condenser must run in a constant downhill pattern. This is easily accomplished, as copper tubing normally comes packaged in a coil. All you have to do to make it into a condenser is stretch the tubing out so that it becomes a spiral with a constant decent. This makes it so that as the steam condenses back into water, it can flow downhill to the catch container.
At that point, there is no need for the system to be sealed, as there is no water vapor, just liquid water.
Such a system can produce many gallons of water a day, depending on how larger you make it and how well you attend it. The fire or other heat source must be constant and the retort filled with water frequently to replace water that has been distilled.
Solar Distillation
The only problem with a standard still is that you need to constantly replenish the fuel for the fire, so that there is enough heat to boil the water and generate steam. While this might not be a problem in an area which is heavily wooded, it could be a problem in areas where there is little firewood to work with. In such cases, a solar still might be a better option.
The basic operating philosophy behind a solar still is the same as that for a normal fire-powered still, with the exception that sunlight provides the heat. Due to this, the overall design of the still is considerably different.
As you can see in this picture, the solar still looks more like a solar oven, than it does the previous still we looked at. The reflectors show on the still above are made of polished aluminum and increase the amount of sunlight entering the cabinet of the still, which is insulated and painted black on the inside, to facilitate absorbing the sunlight and converting it to heat.
Rather than using coiled copper tubing for the condenser, the glass face of the still is the condenser. This is not as efficient as the copper tubing, but it works, as the solar still doesn’t produce as much water vapor as the fire-driven still does, due to a lower internal temperature.
That’s not to say that this still is unable to be used to produce water for a survival situation. I was able to produce about three liters per day of water from it, with a glass surface of about four square feet (2 ft. x 2 ft.). If I were to enlarge it or use multiple units, the still would produce more water.
There were a couple of problems I had with making this sort of solar still. As you can see in the photo above, the glass top is sloped at about a 45 degree angle. This was increased from the first unit I made, which had a 20 degree slope. But that small a slope didn’t work well for causing the condensate to run off the glass, into the catch tube at the bottom. By increasing the angle, I was able to produce more water.
The other problem I had with this design was sealing it properly. It is made predominantly of plywood, which really isn’t designed for making moisture-proof cabinets. My first unit quickly started to delaminate from the water vapor. To compensate for that, this unit was thoroughly sealed and painted with a number of coats of paint, before the insulation was installed on the inside. The door (not visible in the picture) had upgraded seals on it. While I would like to leave the door out altogether, that’s not practical, as it is necessary to clean the pans out occasionally.
The other thing I did to help with sealing was the addition of the PVC tubes shown on the side. These allow water to be added to the unit, without opening it up and losing the heat inside.
Harvesting Salt
If we’re going to be desalinating water to make drinking water, we may as well take advantage of the opportunity to make another valuable commodity as well… salt. During the early years of our country’s history, most of the salt used came from salt licks, so named because animals would go there to lick the salt, so that they might have the salt they needed for survival. Today, few of these naturally occurring salt licks still remain and most salt is mined underground.
Sea salt has become highly prized in modern times, as superior to the normal table salt that we eat. The funny thing about this is a percentage of our normal table salt has always been sea salt, produced by evaporating sea water. They are just packaging it differently now and charging a higher price.
In a post-disaster world, salt will once again become highly prized, due to its rarity. Since salt licks are no longer common, most people won’t have ready access to salt. Yet consumption of small amounts of salt is essential for survival. So, if we are going to be desalinating seawater to produce drinking water, it only makes sense to produce salt for trade goods as well.
The process of distilling seawater is going to leave us with highly concentrated salt water, regardless of which type of still we use. Ideally, we want to stop the still and remove this water, before all of it dries up. That will help prevent damage to our still. The concentrated saltwater we remove will dry faster, leaving behind salt faster than if we used fresh seawater.
The normal way of harvesting salt from seawater is to capture a shallow pool of seawater along the coast, blocking it off from the tides. This water is then allowed to evaporate and the salt left behind is then harvested.
I’ve seen this process done in Mexico, in a coastal area just south of South Padre Island. Full evaporation takes about two weeks, leaving behind a thin layer of salt. With our already concentrated saltwater, the process should be even faster.
I haven’t seen what happens to that salt, between harvesting it and packaging it, but I’ve been curious about it. Since the evaporation process happens over a bed of sand, it seems to me that there would be some sand in the resulting salt; although I haven’t had the opportunity to check it for that. However, if that’s the case, then it would seem to me that the resulting salt would have to be rewetted, the sand separated out and the water to evaporate out again. That’s not real efficient.
In order to do this, we’re going to need to take the concentrated salt water and spread it out, leaving it sitting in the sun to dry. Rather than doing this over a bed of sand, following the traditional method used in Mexico, it seems to me that children’s plastic swimming pools would be just about ideal for drying beds. That would provide us with cleaner salt, as there would be no sand mixed in it.
The other thing I would suggest doing is running the saltwater through a filter, before putting it in the drying beds. Simple coffee filters or something similar would be sufficient for this filter stage. Adding in this step would filter out any solids floating in the water, while allowing the dissolved salt and other minerals to pass through. The resulting salt could be used directly, without any further processing. While the grain of the salt would be larger, like commercial “sea salt,” it would still be usable. ‘
One final point I want to make about this salt is that it would be perfectly safe from any bacteria or other microscopic pathogens. The process of drying the salt means that any bacteria that might be in the water would be dried as well, killing them and eliminating any danger they might cause.












tom james | May 7, 2019
|
Water is an amazing solvent…and distilled water is hypotonic, meaning it is an exceptionally good solvent. This effect won’t matter much over days or weeks, but, drinking distilled water over long periods will leach beneficial minerals out of your body, to a harmful degree.
If relying on distilled water, consider taking regular mineral supplements…or use a lot of sea salt on your food!
Rocky | April 30, 2020
|
Something that has concerned me about sea salt is all the heavy metals that are dissolved in sea water.
joe | October 2, 2020
|
You are right, and wrong at the same time. Yes, distilled water could extract minerals, but only the inorganic ones, which the body can not use as we are not plants, only plants can use inorganic minerals. That is why people that live in areas that have lots of minerals in their water have so many problems. The body has a hard time getting rid of all those minerals. We, as animals can only use organically bound minerals, whi
ch we get from our food.
Dennis | April 24, 2021
|
You get enough minerals out of you’re food and distilled water doesn’t leach minerals from your body ,that’s an old wife’s tale I have been drinking distilled water exclusively for over 40 years and I’m not sick or dying from doing so.
Dale | May 7, 2019
|
A still, whether powered by fire or sunlight, should also serve to purify fresh water, would it not? My question is whether there is a danger that the pollutants in the water would evaporate along with the water and be condensed back into the distilled water so that it remained polluted?
What about water that is polluted by chemicals and/or organic volatiles such as gasoline, diesel, oil, etc.? Would the chemicals or volatiles be re-condensed into your distilled water?
How about water that has been polluted by sewage? The heat of the distillation process should kill any bacteria, especially in a fire-powered still. What about the other nasty things in sewage? Is there any danger of them ending up in your distilled water?
Sam | May 7, 2019
|
Good question. Anything that vaporizes above 212* will be left behind. Your volatiles, by definition, will not be. If that is a concern where you are (or will be), I would agitate the water first, to allow for evaporation of those chemicals. Actually, I would filter it first, to remove most of the solids, then agitate. You don’t want to break up any solids into particles your simple filter won’t catch. If you have the means, boil your water in the open, then filter and distill. There is no one size fits all in a survival situation, so see through your own eyes. Bill is an excellent source of information, as are others on this site. But the squeemish won’t make it.
tom james | May 7, 2019
|
Using an activated charcoal filter in the above step would help to remove any contaminating chemicals.
Bill in Idaho | May 2, 2020
|
A Good Commentary, Sam. I would be using Both – a 10 Micron Solids Filter – Followed by an Activated Charcoal (Carbon) Filter – Use a “Flow Rate” No More Than 1-2 Quarts in 10 minutes. Your Organic Volatiles, and nearly All of your Inorganic Volatiles will be Gone then. Now you can distill the output water with confidence.
tom james | May 7, 2019
|
Seems to me there would always be the chance for microbial cross-contamination within the interior of the distillation chamber….dirty water could splash into the discharge chute, algae could grow in the chute, etc etc.
If you’re concerned about it, you could always subject the distilled water to a final SODIS purification step after it is discharged from the still. Google it for more info.
Sam | May 7, 2019
|
Thanks, Tom. Sunlight has long been known for its purification properties. I like the info on the SODIS site. I’ll follow up on that. Splash could be an issue, if you boil your water vigorously. If you have little control over your rate of burn (heat source), you might figure out some kind of splash guard for your outlet. With clean condensate going through your copper tubing, I don’t think algea,, or any other growth that needs sunlight, would be a concern.
joe | October 2, 2020
|
Not much of a problem, the chemicals that have a lower temperature evaporation point will come out first, so if you don’t start collecting the water until the water droplets start coming out they will all be gone. As long as you stop collecting the water before it has all evaporated, then those chemicals, pollutants that have a higher temperature evaporation point will not come out of the solution. So distillation is an excellent method of purifying your water.
Sheree Lally | May 7, 2019
|
I wondered about that as well.
Dale | May 7, 2019
|
Thanks, Sam. Good ideas. I would filter any water as a matter of course, but I hadn’t thought about agitating it to allow volatiles to evaporate before distilling. Boiling in the open, then filtering and distilling is also good.
Dale | May 7, 2019
|
Thanks, Sam. Good ideas. I would filter any water, first, before distilling, as a matter of course. But, I hadn’t thought of agitating the water first to allow chemicals and volatiles to evaporate. Boiling in the open air, then filtering and distilling is also good.
Dale | May 7, 2019
|
Thanks to Tom, too. I’d never heard of the SODIS method. Simple and elegant.
Grammyprepper | May 8, 2019
|
The comments above answered several questions Ihad, so thank you all. Two issues not addressed: radioactivity, which we all know continues to leak from Fukishima, and prescription medication residues (which may be more of an issue in interior water tables than ocean water)..
j | October 3, 2020
|
distilling will remove it, and everything else. boil open first to get rid all that have a lower boiling point than water and stop collecting before all the water have boiled off and you won’t have any problem with the purity of your water.
Dale | May 8, 2019
|
Grammyprepper, I would think that charcoal filtering would catch any radioactive particles as well as the residues of prescription medications that end up in sewage and from there into streams, rivers and water tables. However, I’m not sure. If anyone has any concrete knowledge about the effectiveness of charcoal filtering on those two, it would be appreciated.
Grammyprepper | May 9, 2019
|
Thanks Dale, I also was thinking charcoal filtering regarding of purification method. But some hard facts would be nice, if anyone has them or time to research them!
Grammyprepper | May 9, 2019
|
oops, meant REGARDLESS of purification method
j | October 3, 2020
|
best to distill,
distilling will remove it, and everything else. boil open first to get rid all that have a lower boiling point than water and stop collecting before all the water has boiled off and you won’t have any problem with the purity of your water.
Ljb | September 14, 2019
|
To make sure you get mostly water use a thermometer if you only want water. allow the evaporated product to vent to the atmosphere until it reaches 212 degrees (at sea level) this will allow volatile compounds with an evaporation point lower then water to vent to the atmosphere before you start condensing same goes once you start condensing watch the temperature if it gets above 212 lower the temperature to keep compounds with a higher evaporation point from entering your system
All of this is at sea level where sea water is most likely found temperature will need to be adjusted for different elevations and pressures
Bianca Wall | September 15, 2019
|
Dale & Everyone….After the catastrophe in Japan, Dr. Carl ….? who owns the Seychelle Co. invented a Water Filter to take out the radiation. It was tested at 2 independent labs in Calif. who stated that Seychelle’s filters took out 99.8% of the particulates. I believe this particular filter is called the Extreme Water bottle. The Army is one of his customers.for various other water filters Seychelle sells. .
Bob Hodges | September 15, 2019
|
Like all of us, I hope it never comes to the need to have to desalinate but we need to prepare and the methods described are very informative. Ignorant as I am, I wondered if there is any potential to using old car/truck radiators, refrigerator/freezers, old home heater radiators, or other devices that can be adapted or stripped of useful parts? At best, I offer my question as “food for thought” to those more mechanically and technically inclined than me which is just about everybody.
Michael Schneider | March 27, 2020
|
Old auto radiators use lead solder. That will blind or kill you.
William M Doherty | October 4, 2020
|
Newer radiators use mostly plastic and aluminum. Would the aluminum leach into the water?
Larry | November 3, 2019
|
I saw a documentary film that showed how seawater was concentrated in large pools (similar to the operation the author saw in Mexico). The film was black and white and took place in South America, possibly Argentina, in the early 1930s as best as I can remember. It was amazing how much salt was produced with manual labor. Sheets of salt were removed from the bottom of the evaporation pond and placed in the bottom of a wooden boat. The salt in the boat was wetted with the high salinity water from the pond to wash it before removing it from the boat and spreading it to dry. It was then weighed to determine how much the people who produced it would be paid. Then the salt was carried to the top of a huge mound of salt by a member of the crew (usually family members) and dumped on the top of the mound. The salt was put into burlap bags for shipment and carried by a powered boat to a port. I don’t remember the name of the film. I saw it on TV and wish I had saved the recording on DVR. This description can’t accurately describe the process and the hard lives of those who worked to produce the salt.
joe | October 3, 2020
|
They are still doing it that way in the Philippines, cermet tiles are lining the pounds and the evaporated salt is scraped off the tiles.
Wayne Wilhelm | December 28, 2019
|
One gallon of sea water will product approximately 5 ounces of salt. I filter the water first, then separate it into several pans on the stove. As it reduces down, I combine it into one pan to finish the evaporation and scrape the salt from the pan.
Patricia | November 12, 2021
|
I intend to try this, but without the plastic on the baby pool which is not food grade. I have been considering this issue for a while because I am in Southern California-plenty of beaches, no water. Anyone have any suggestions on what other materials could be used? Why not just roasting pans from the kitchen? I already have some so they are readily available. Is the flexibility from the baby pool why that was recommended or just the size? I will also check for radiation. I have a Geiger counter. My fear is that any radiation will end up in the salt because of its weight. Thanks for any advice you can give.